all heart
Houston company behind game changing artificial heart gets FDA green light
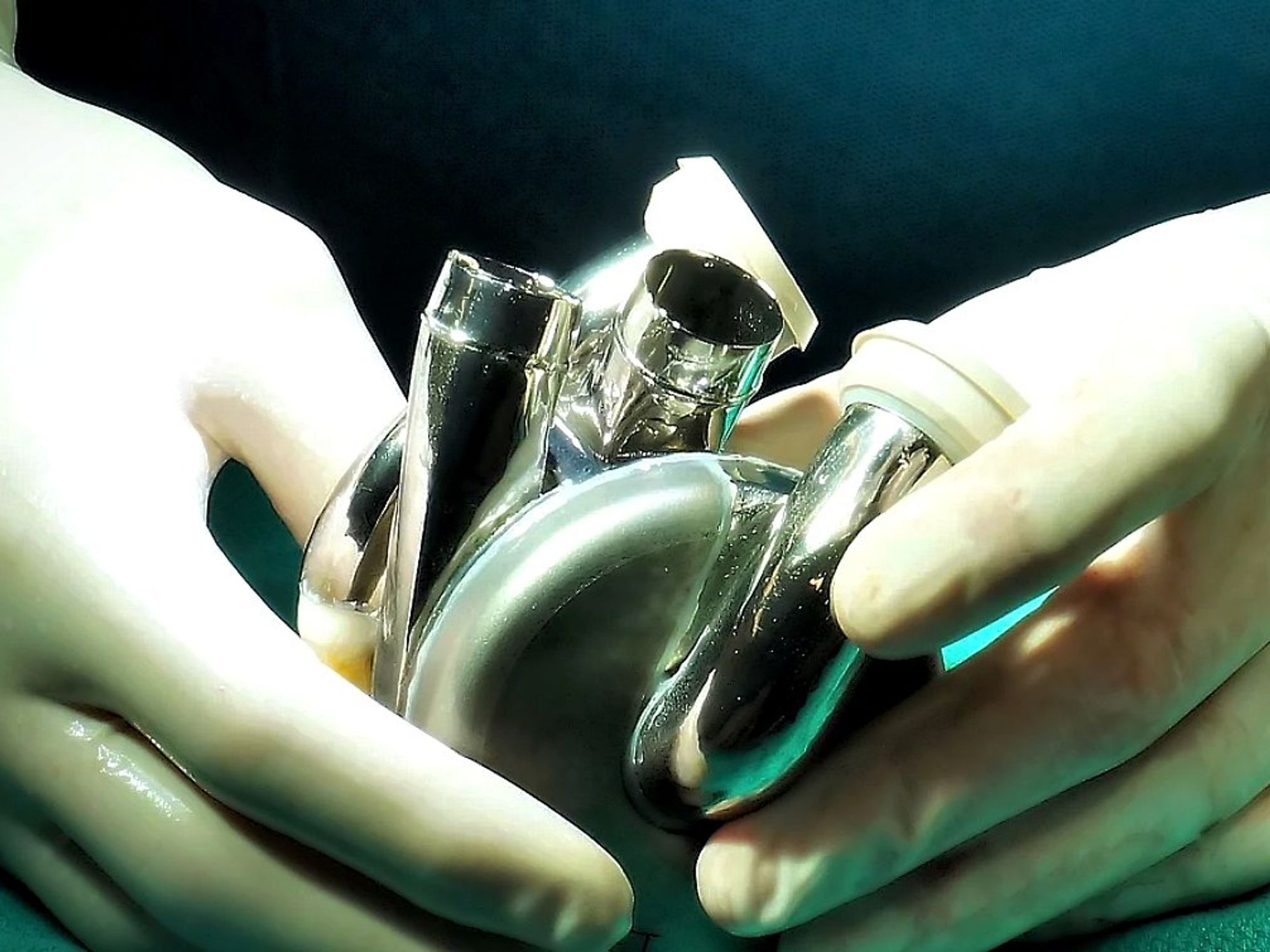
Houston company with a breakthrough heart health tech has received a green light from the FDA.
BiVACOR, a Houston-headquartered medical device company, has received FDA approval for its Total Artificial Heart (BTAH) IDE first-in-human early feasibility study (EFS). The BTAH device itself is designed to take over all function for patients with heart failure. The BTAH is roughly the size of a human fist, which means that, while it could support an active adult male, it may also fit many women and children.
Led by CEO Thomas Vassiliades, a former heart surgeon, BiVACOR is based on a system of magnetic levitation. “Our pump is just one moving impeller that sits in the middle of the housing where the blood is. Imagine an artificial heart — the container that has your blood — and the device spinning in the inside — basically a wheel spinning your blood to the rest of your body. The device is suspended by magnets — it's not touching anything,” Vassiliades told InnovationMap in a podcast earlier this year.
Because of that, BiVACOR could potentially last for a patient’s entire life with no wear — something, Vassiliades explains, is new to the field.

The EFS includes 10 hospitals enrolled as possible sites and is slated to begin in 2024. One location is Houston’s own Texas Heart Institute.
-----
Continue reading this story on our sister site InnovationMap.
